The blowtorch experiment shows that electrons can and do carry significant amounts of heat (or cold) because electric current involves a lot of electrons, perhaps Avogodro’s number of electrons, passing by each point in the wire every minute.
In the blowtorch experiment , the idea of temperature starts with the idea of heating a copper wire until the wire is cherry hot. The wire glows red. The glow comes from the copper atoms bouncing around with heat. At first the idea of a hot temperature would show that the wire would glow with a continuum of frequencies. All of the red colors would be expected to be seen in the spectrum of color from the glowing wire.
A closer look at the spectrum reveals some details about the atomic structure of s copper wire. Copper can glow with some red colors, but not others. We are asked to believe that the wire is made of copper atoms which can vibrate with some frequencies and not others. Frequency of oscillation makes the colors of light emitted from the hot solid. In this case the solid copper is opaque. Red light from heat doesn't go through the solid as light. Rather this heat energy goes through the opaque solid as vibrations or noise. The cherry hot copper is very loud inside, and the loud sounds from heat come out as cherry red light on the surface. Since the copper atoms can vibrate with some frequencies and not others, the actual spectrum looks more like a comb with the tines pointed up.
An electron that has been in the solid wire for a long time may be oscillating in it's own internal structure with the same temperature as the wire, but the electron can have temperature A, temperature C, or temperature E. However the electron cannot have temperature B, or temperature D, or temperature F. These frequencies aren't available in the wire, so an electron that is at the same temperature as the wire cannot have temperature B, D, or F.
Compare this with the frequency spectrum of the transistor or diode made of silicon. The tines of the silicon spectrum don't line up with the copper spectrum. Silicon can have temperature B, temperature D, or temperature F. Silicon cannot have temperature A, or temperature C, or temperature E. These frequencies aren't available in the silicon transistor, so the electron can only take on temperature B, D, or F as a result of passing through the solid silicon.
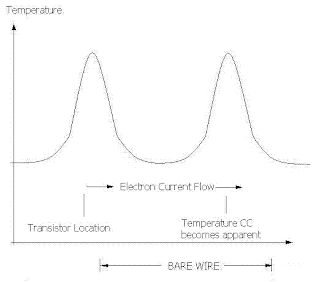
A silicon transistor in a working circuit becomes hot because the circuit has been working for a long time. Transistors get hot as a natural function of regulating electric current flow. The hot ransistor has temperature BB, DD or FF. After being squeezed through the NPN junction of a hot transistor, the electron heats up. The electron will come out of the hot transistor with temperature BB, DD or FF. Temperature AA, CC, EE aren't available in the solid silicon. As the electron moves along with drift velocity in the silicon, it has intimate contact with the silicon material that vibrates with heat, but only with those temperatures such as BB, DD, or FF. The electron goes through the hottest part of the transistor, namely the PN junction.
Temperature BB, DD, or FF won't go through solid copper. After the hot electron leaves the silicon transistor and moves into the copper wire then the electron's temperature becomes hidden. The electron's temperature becomes hidden because the precise "frequency or temperatue" won't go though copper. The electron vibrates the electron is hot. Those hot vibrations, from silicon, won't go through copper.
Later on in time, which also means somewhere farther along the wire as the electrons move along, those electrons may cool off a little because of collisions with other electrons. And then the heat energy of the electron becomes apparent to the outside world. A hot electron with temperature CC will eventually cool off to have temperature BB. Then the vibration of the electron that represents temperature BB will go through the copper wire and then the heat energy will leave the copper wire as red or infra red light.
In the circuit diagram, room-temperature electrons are going into the silicon NPN transistor. The NPN transistor heats up and puts out hot electrons. If there is another transistor or diode near the first transistor, then electrons with temperature CC will get into the next transistor or diode. If those hot electrons get in to the PNP transistor, which is made of silicon as well, then the silicon of the PNP transistor can conduct the exact vibration frequency temperature CC so that the heat energy tends to heat up the PNP transistor a lot.
Key point: The PNP tramsistor absorbs heat enery from the NPN transistor. Then the PNP transistor adds more heat. As a result the PNP transistor is usually the first to fail.
The designer would wish to add a diode to the amplifier circuit, to prevent heat energy from the NPN transistor from getting in to the PNP transistor. This design will add "diode B" to protect the PNP transistor from hot electrons coming out of the NPN transistor. The diode will behave as a thermalizing junction. The thermalizing junction "Diode B" should be mounted to the same heat sink to which the NPN and PNP transistors are mounted. The physical size of "Diode B" must be large enough to handle the heat energy more so than the electric current. "Diode B" should be made of the same material as the NPN transistor, perhaps in the same size package for easy mounting to the heat sink. Heat sink not shown in this diagram.
The blowtorch experiment shows that electrons can and do carry significant amounts of heat (or cold) because electric current involves a lot of electrons, perhaps Avogodro’s number of electrons, passing by each point in the wire every minute.




No comments:
Post a Comment
Moderated comments should be rated PG.